March 23rd 2021
Vegetable starch and carbon nanotubes form the electrodes of a 3D-printed lithium-ion battery that promises a more environmentally-friendly, higher-capacity source of power for mobile devices.
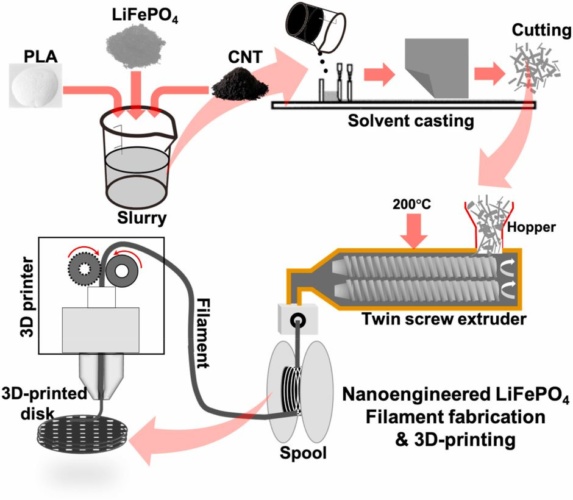
This is the claim of engineers led from Glasgow University who have been looking to make lithium-ion batteries capable of storing and delivering power more efficiently and sustainably. The battery’s design and fabrication is outlined in a paper published in the Journal of Power Sources.
Lithium-ion batteries comprise a positive electrode, often made from lithium cobalt/manganese oxide or lithium iron phosphate, and a negative electrode, often made from lithium metal. During charging, lithium ions flow through an electrolyte from the positive electrode to the negative electrode. During use, the ions flow in the opposite direction.
The thickness of the electrode can limit the battery’s performance. According to Glasgow University, thicker electrodes restrict diffusion of lithium ions across the electrode, thereby limiting the specific energy of lithium-ion batteries. Increasing electrodes’ thickness also decreases their strain-tolerance, making them more prone to cracking and rendering them useless.
The Glasgow-led team’s battery has introduced nanoscale and microscale pores into their design. By covering the surface and interior of the electrodes with pores, they can greatly increase the surface area compared to a solid electrode of the same external dimensions.
To do so, they used an additive manufacturing technique to tightly control the size and placement of each and every pore in their electrodes. They loaded their 3D printer with a material they developed which combines polylactic acid, lithium-iron phosphate and carbon nanotubes. The polylactic acid is a biodegradable material processed from the starch of corn, sugar cane, and sugar beet, increasing the battery’s recyclability.
They experimented with making circular electrodes at three different thicknesses of 100, 200 and 300 microns. Each electrode was tested with different combinations of materials, varying the amount of carbon nanotubes in the material mixture from 3 to 10 per cent by weight, and the porosity from 10 to 70 per cent by introducing tightly-controlled grids of holes throughout the electrode.
The team’s 300-micron electrode battery with 70 per cent porosity performed the best during testing, with a specific capacity of 151mAh/g, which Glasgow University said is two to three times the performance of a traditional lithium-ion battery with a solid electrode of the same thickness.
The increased porosity of the thickest 300-micron electrode also influenced the battery’s areal capacity. The thicker electrode was capable of storing 4.4mAh cm−2 compared to 1.7mAh cm−2 achieved in the 100-micron electrode, a gain of 158 per cent.
The research was led by Dr Shanmugam Kumar from Glasgow University’s James Watt School of Engineering, alongside colleagues from Khalifa University of Science and Technology in Abu Dhabi, and Texas A&M University and Arizona State University in the USA.
In a statement, Dr Kumar said: “Lithium-ion batteries are increasingly common in everyday life and are likely to continue to increase in ubiquity as we move towards more electrification of transport and a more sustainable world. However, lithium-ion batteries have their own sustainability issues, so it’s important that we look to find new ways to make them better and more environmentally-friendly.
“The 3D printing process we’ve used in this research gives us a remarkable amount of control over the electrodes’ porosity, allowing us to engineer very precisely a new metamaterial capable of addressing some of the shortcomings of the current generation of lithium-ion batteries. We’ve created a battery with a high specific capacity and areal capacity with excellent cyclability.
“These are promising initial results, and we’re keen to continue to explore the possibilities that this kind of microarchitected materials offer to create better, more recyclable batteries for future consumers.”
Source: https://www.sciencedirect.com/science/article/abs/pii/S0378775321001701?dgcid=author
